Difference between revisions of "Determination of Planck's Constant"
(Adição da secção de links) |
|||
| Line 4: | Line 4: | ||
=Experimental Apparatus= | =Experimental Apparatus= | ||
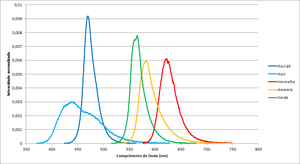
| − | [[File:Espectro_dos_leds.png|thumb|alt= | + | [[File:Espectro_dos_leds.png|thumb|alt=Led's spectrum|Figure 1: Led's spectrum.]] |
| − | The photoelectric cell is from the apparatus PASCO AP-9368. It works like a capacitor where | + | The photoelectric cell is from the apparatus PASCO AP-9368. It works like a capacitor where one of the plates emits photoelectrons when excited by light. |
| − | The potential of the photocell | + | The potential between the plates of the photocell will increase with the emitted photoelectron accumulation. After reaching a certain voltage, the stopping potential, no more photoelectrons will have enough energy to reach the second plate. This voltage will depend on the wavelength of the incident light (photon energy). |
After each experiment the photocell is connected to ground to discharge it. | After each experiment the photocell is connected to ground to discharge it. | ||
| − | The leds have different | + | The leds have different efficiency leading to different intensities for chosen brightness. Therefore, the charging time will be different between colors. |
{| border="1" style="text-align: center;" | {| border="1" style="text-align: center;" | ||
| Line 48: | Line 48: | ||
=Protocol= | =Protocol= | ||
| − | The number of photoelectrons emitted will increase with the intensity of light | + | The number of photoelectrons emitted will increase with the intensity of light (corpuscular behaviour of light). |
| − | #Choose a led to light | + | #Choose a led to light upon the photocell |
| − | #Measure the stopping potential. | + | #Measure the stopping potential. Note the time necessary to reach the maximum potential. |
#Repeat step 2 for different intensities. | #Repeat step 2 for different intensities. | ||
| Line 89: | Line 89: | ||
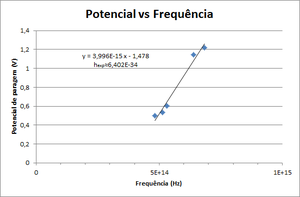
| − | [[File:Constante de Planck.png|thumb|alt= | + | [[File:Constante de Planck.png|thumb|alt=Planck's constant|Figure 2: Potential vs. Peaks Frequency of the spectrum ]] |
| − | The | + | The photoelectron's kinetic energy depends only on the frequency of the incident light. If the frequency of light increases, the energy will increase. |
#Obtain the stop potentials for different colour leds. | #Obtain the stop potentials for different colour leds. | ||
Revision as of 21:40, 18 March 2013
Contents
Description of the Experiment
The purpose of this experiment is to study the photoelectric effect and calculate Planck's constant using 5 different coloured leds and a photoelectric cell.
Experimental Apparatus
The photoelectric cell is from the apparatus PASCO AP-9368. It works like a capacitor where one of the plates emits photoelectrons when excited by light. The potential between the plates of the photocell will increase with the emitted photoelectron accumulation. After reaching a certain voltage, the stopping potential, no more photoelectrons will have enough energy to reach the second plate. This voltage will depend on the wavelength of the incident light (photon energy).
After each experiment the photocell is connected to ground to discharge it.
The leds have different efficiency leading to different intensities for chosen brightness. Therefore, the charging time will be different between colors.
| Color | Frequency (THz) | Wavelegth (nm) | Espectros dos leds |
|---|---|---|---|
| Blue.ab | 638.7 | 469.70 | File:Espectro Azul.ab.txt |
| Blue | 684.6 | 438.20 | File:Espectro Azul.txt |
| Red | 482.2 | 622.21 | File:Espectro Vermelho.txt |
| Yellow | 514.4 | 583.16 | File:Example.txt |
| Green | 530.8 | 565.22 | File:Espectro Verde.txt |
Protocol
The number of photoelectrons emitted will increase with the intensity of light (corpuscular behaviour of light).
- Choose a led to light upon the photocell
- Measure the stopping potential. Note the time necessary to reach the maximum potential.
- Repeat step 2 for different intensities.
| Color #1 __________(name) | Intensity (%) | Stop Potential (V) | Time (s) |
|---|---|---|---|
| 100 | |||
| 80 | |||
| 60 | |||
| 40 | |||
| 20 |
The photoelectron's kinetic energy depends only on the frequency of the incident light. If the frequency of light increases, the energy will increase.
- Obtain the stop potentials for different colour leds.
- Draw a graphic of Stop Potential vs Frequency. Fit it to \( V = \frac{h}{e} \nu - \frac{W_0}{e} \) and obtain Planck's constant.
| Colour (name) | Frequency (Hz) | Stop Potential (V) |
|---|---|---|
Advanced Protocol
Under construction.