Difference between revisions of "Determination of Planck's Constant"
| Line 17: | Line 17: | ||
{| border="1" style="text-align: center;" | {| border="1" style="text-align: center;" | ||
| − | |+ Table 1 – Led's spectrum peaks | + | |+ Table 1 – Led's spectrum peaks; these values should be used considering the wavelength's dispersion with 7 mA. |
|- | |- | ||
!Color | !Color | ||
| Line 49: | Line 49: | ||
||[[File:Espectro_Verde.txt|Green]] | ||[[File:Espectro_Verde.txt|Green]] | ||
|} | |} | ||
| − | |||
=Protocol= | =Protocol= | ||
Revision as of 15:07, 28 October 2013
Contents
Description of the Experiment
The purpose of this experiment is to study the photoelectric effect and calculate Planck's constant using 5 different coloured leds and a photoelectric cell.
Experimental Apparatus
The photoelectric cell is from the PASCO AP-9368 apparatus. It works like a capacitor where one of the plates emits photoelectrons when excited by light. The potential between the plates of the photocell will increase with the emitted photoelectron accumulation. After reaching a certain voltage, the stopping potential will be greater than the photoelectron's kinetic energy, and these will not have enough energy to reach the second plate. This voltage will depend on the wavelength of the incident light (photon energy).
After each experiment the photocell is connected to ground to discharge it.
The leds have different efficiency, leading to different intensities for a chosen brightness. Therefore, the charging time will be different between colors.
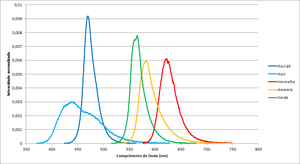
The graph in figure 1 shows each led's spectrum, table 1 having more details.
Please note that the wavelength for each led depends on the junction's temperature, and this will depend not only on room temperature, but also on the current passing through the junction. This effect (red shift) is more noticeable in warm-coloured led (green-red) than blue, so the calculation of Planck's constant will suffer a shift.
| Color | Frequency (THz) | Wavelegth (nm) | Espectros dos leds |
|---|---|---|---|
| Blue.ab | 638.7 | 469.70 | File:Espectro Azul.ab.txt |
| Blue | 684.6 | 438.20 | File:Espectro Azul.txt |
| Red | 482.2 | 622.21 | File:Espectro Vermelho.txt |
| Yellow | 514.4 | 583.16 | File:Example.txt |
| Green | 530.8 | 565.22 | File:Espectro Verde.txt |
Protocol
The number of photoelectrons emitted will increase with the intensity of light (corpuscular behaviour of light).
- Choose a led to light upon the photocell
- Measure the stopping potential. Note the time necessary to reach the maximum potential.
- Repeat step 2 for different intensities.
| Color #1 __________(name) | Intensity (%) | Stop Potential (V) | Time (s) |
|---|---|---|---|
| 100 | |||
| 80 | |||
| 60 | |||
| 40 | |||
| 20 |
The photoelectron's kinetic energy depends only on the frequency of the incident light. If the frequency of light increases, the energy will increase.
- Obtain the stop potentials for different colour leds.
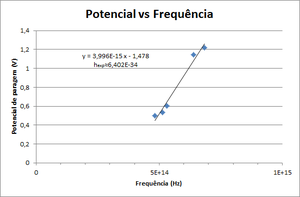
- Draw a graphic of Stop Potential vs Frequency. Fit it to \( V = \frac{h}{e} \nu - \frac{W_0}{e} \) and obtain Planck's constant.
| Colour (name) | Frequency (THz) | Stop Potential (V) |
|---|---|---|
Advanced Protocol
- Study the photocell's charging process for different intensities.
- Find the expected value for wavelength using the led spectra.
- Use those values for a new graphical fitting and compare the results.
- Redo the voltage vs. frequency graph, this time with error bars.
Note: This setup uses a 12bit ADC from 0V to 5V.
Theoretical Principles
Photoelectric effect
The photoelectric effect happens when a metal surface is illuminated by a light with a given frequency, causing electrons to be freed. When a photon with energy \( E \) collides with a metal, it transfers it's energy to an electron in the crystal structure. The emission of electrons is deeply connected to the frequency of the light shone upon the surface. For each metal there is a critical frequency, \( \nu _0 \). If incident radiation has frequency bellow \( \nu _0 \), then there are no emitted electrons. If the former is above the latter, then the kinetic energy of the emitted electrons is proportional to the energy of the photons. The light's intensity changes only the number of emitted photoelectrons, not it's energy (this goes against what is expected in the classical theory of radiation).
Einstein proposed the following explanation: light is made of photons with a given energy, proportional to frequency (\( \nu \)):
\[ E = h \nu \]
Where \( h \) is Planck's constant. The photoelectric effect is, essentially, a collision between a photon (light) and an electron (in the metal) where the former gives all it's energy to the latter. Since the electron's energy is higher when in vacuum (as opposed when it's bound in the metal's crystal structure), the electron is only freed if the photon's energy is higher than the difference between the electron's energy in vacuum and in the metal (figure 1).
UNDER CONSTRUCTION.
In This Setup
UNDER CONSTRUCTION.
Historical Elements
In 1921, Albert Einstein won the Nobel Physics Prize for his work on the photoelectric effect.